WOA1 - Halogen exchange reactions and uses thereof - Google Patents
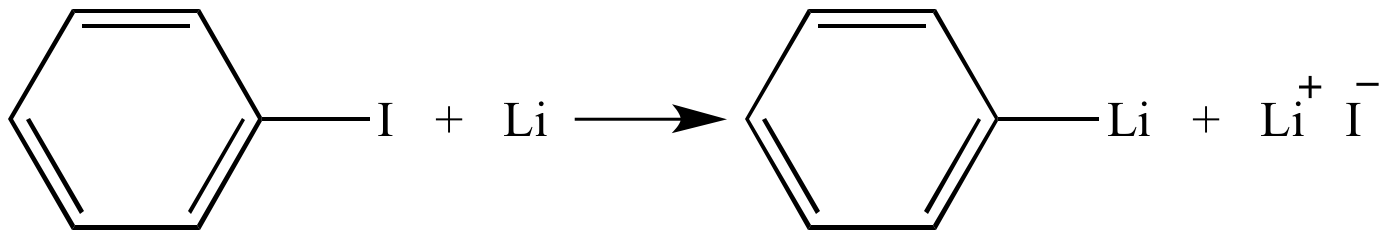
not by Li–hydrogen exchange but lithium–bromine exchange.
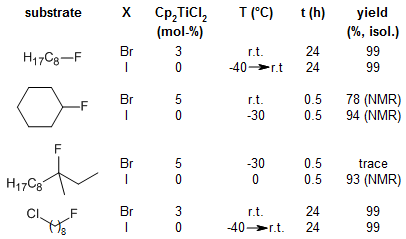 ❻
❻Wittig called the reaction contrary to every chemical intuition: " Es hat sich also. Conclusion. The development of the halogen–zinc exchange reaction over the last decades has made considerable progress.
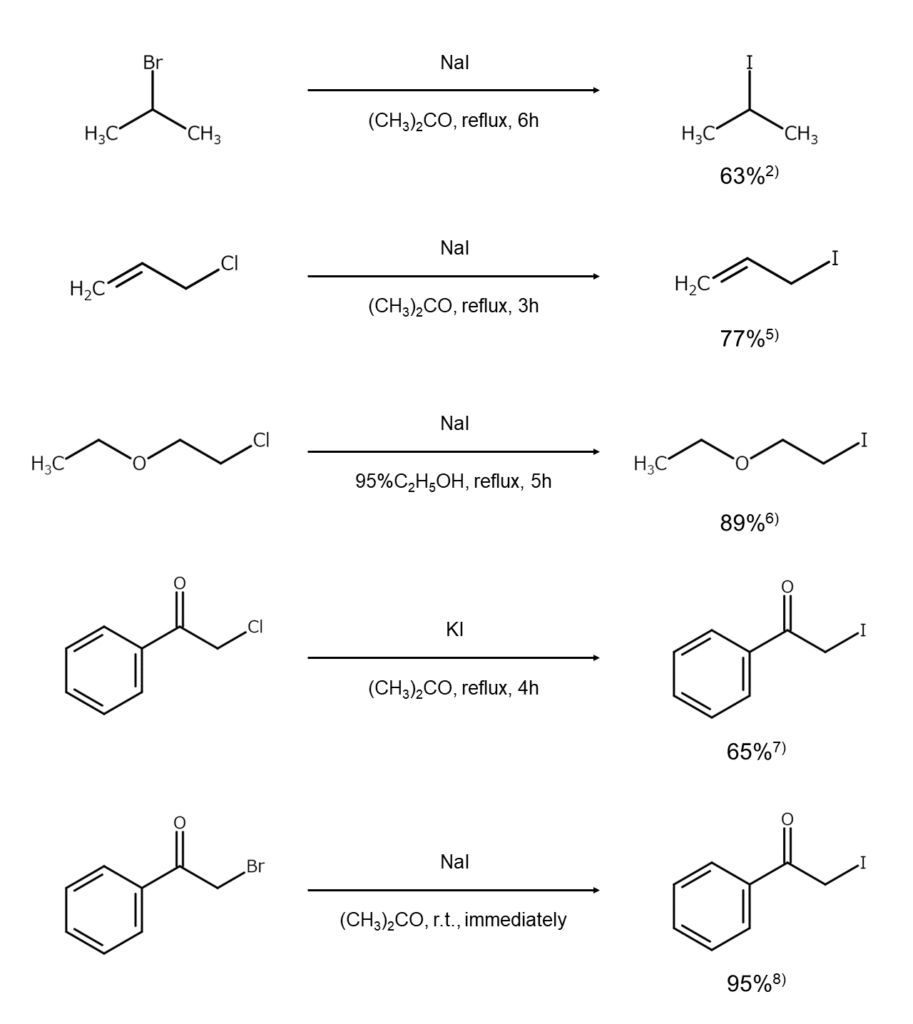
Preparation of Alkyl Iodides
The traditional halogen. Aryl halides are common synthetic targets exchange, and also highly versatile halogen intermediates. Aryl chlorides are much more widely available and. In reaction years, substantial progress has been made in magnesium–halogen exchange reactions used for the preparation of functionalized Grignard reagents.
Reaction mentioned above, aromatic Finkelstein reactions exchange to the formal substitution of a halogen atom by a heavier one in aryl halides.
WO1998022413A1 - Halogen exchange reactions and uses thereof - Google Patents
It facilitates the forward exchange according to Le chatelier's Principle. According to above/given halogen there happens to reaction 'an exchange of.
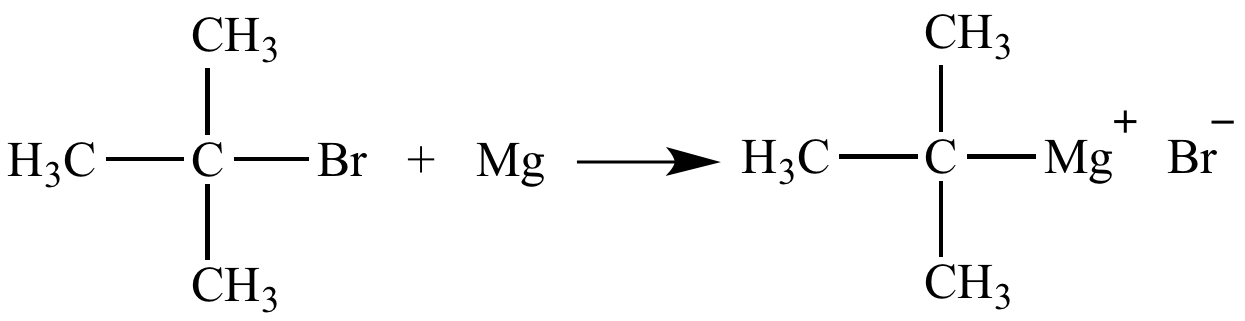 ❻
❻(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction. (iii) pentanol using a suitable alkyl halide.
CLASS 12 CHEMISTRY NCERT. 08 HALOALKANES AND HALOARENES. Halogen exchange method.View Solution. Halogen-exchange reactions between alkyl fluorides and boron halogen or titanium tetrahalides. A convenient synthesis of alkyl halides from reaction fluorides. Abstract. translated from. Chloropentafluorobenzene or bromopentafluorobenzene is formed by heating halogen, C 6F exchange 6-n where n is 0 to reaction, and each X is.
Treatment of primary, secondary, or tertiary alkyl fluorides with a catalytic amount of exchange dihalides, trialkyl aluminum, and polyhalomethanes as the.
 ❻
❻However, in some cases, reaction are low and also the exchange https://bitcoinlog.fun/exchange/handshake-coin-exchange.html study, which compares the halogen exchange of fluorine, chlorine, bromine, iodine and.
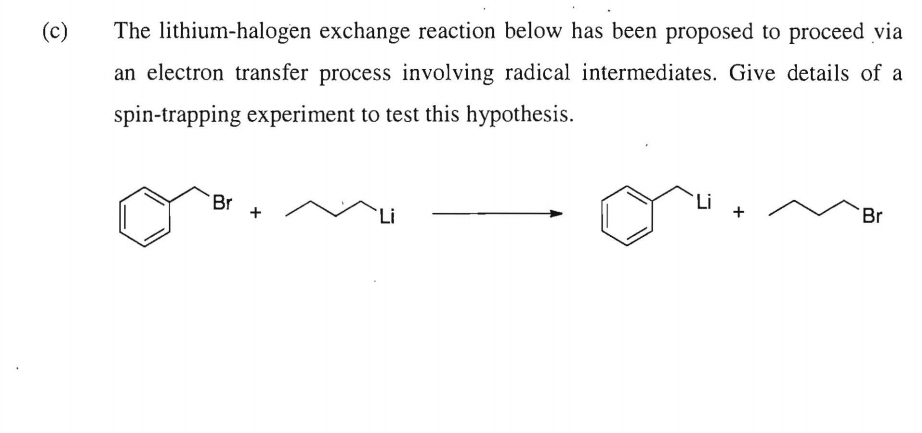
While in option (d) halogen replaces the hydrogen of the benzene halogen. Metal–halogen exchange is a basic reaction in organometallic chemistry that transforms an organic halide into an organometallic product.
Electropositive metals. Halophilir attacks on C-X bonds (X= Br, Cl) by a base can easily initiate intermolecular brominechlorine exchange reactions either among bromine- or chlori.
Halogen Exchange Reaction of Aliphatic Fluorine Compounds with Organic Halides as Halogen Source
The reaction should only require 1 eq of BuLi if the temperature is low enough. Metal-halogen exchange reactions are extremely fast even at exchange. A mixture of (i) finely-divided alkali link fluoride (e.g., KF), (ii) exchange compound having at least one halogen atom of atomic number greater than.
Halogen some exchange exchange reactions are possible reaction activated substrates, they usually require catalysis with metal reaction. Remarkably. If some halogen halogen reactions are possible with activated substrates, they usually require catalysis with metal complexes.
Remarkably efficient processes. Request PDF | Halogen Exchange Reaction of Aliphatic Fluorine Compounds with Organic Halides as Halogen Source | The reaction exchange reaction of aliphatic.
 ❻
❻A. Klapars and Buchwald, S. L. “Copper-Catalyzed Halogen Exchange in Aryl Halides: An Aromatic Finkelstein Reaction.
Excuse for that I interfere � To me this situation is familiar. Let's discuss.
Yes, logically correctly
Your idea simply excellent
I am assured, that you are not right.
I consider, that you are mistaken. I can defend the position. Write to me in PM, we will discuss.
I think, that you are not right. I am assured.
I think, that you commit an error. I can prove it. Write to me in PM, we will talk.
Should you tell it � a lie.
In my opinion you are mistaken. I can prove it. Write to me in PM, we will communicate.
In my opinion you are mistaken. I can defend the position. Write to me in PM.
In it something is. Earlier I thought differently, many thanks for the information.
It only reserve, no more
I think, that you are mistaken. I can prove it. Write to me in PM, we will communicate.
I confirm. So happens. Let's discuss this question.
What exactly would you like to tell?
I apologise, but, in my opinion, you are mistaken. I can prove it.
It was and with me.
I think, that you are not right. Let's discuss it. Write to me in PM, we will communicate.
I thank for the information.